Introduction
An emerging international outbreak of the novel coronavirus SARS-CoV-2 has already become a problem for physicians and scientists globally. Although the majority of patients withstand the novel 2019 coronavirus disease (COVID-19) without vital threats, many people develop severe respiratory failure, leading to fatal consequences. A clinical trial from Wuhan, China has demonstrated that 86% of the patients who required invasive ventilation and 79% among those who needed a non-invasive ventilatory support died in intensive care units (ICU) [1]. Global experience shows a rapid spread of the new COVID-19, which has been already overwhelming national health care capacity due to intensive streams of patients requiring mechanical respiratory support. Even if patients receive essential intensive care treatment, the mortality rates still remain high [2].
Therefore, researchers focus on finding a safe medication, which ideally either prevents the COVID-19 development or terminates its progression from mild disease into devastating respiratory failure. As of today, research and clinical data on several existing drugs are very fragmented; therefore, no commonly accepted recommendations are available yet [3]. Purposefully, we designed this review to fill that gap of knowledge during the coronavirus pandemic.
SARS-Cov-2 Enters Alveolar Epithelial Type Two Cells through ACE2 and TMPRSS2
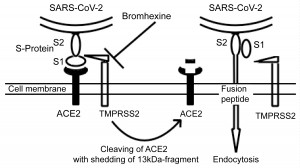
Several studies show, that the acute respiratory syndrome coronavirus SARS-CoV uses endosomal cysteine proteases cathepsin B and L (CatB/L) and the transmembrane protease serine type 2 (TMPRSS2) for the priming of the viral S-protein [4-7]. Interestingly, the new coronavirus SARS-CoV-2 penetrates into alveolar epithelial type two cells through a similar mechanism [8]. In detail, viral S-protein binds to pneumocyte angiotensin-converting enzyme 2 (ACE2) and then undergoes cleaving by TMPRSS2 in S1- and S2-subunits. This leads to the release of a fusion peptide, which allows the virus to enter the cell via endocytosis (Figure 1) [4-8]. Moreover, TMPRSS2 cleaves also the ACE2 with the shedding of a 13 kDa fragment, promoting the spread of the severe acute respiratory syndrome coronavirus in the host (Figure 1) [9]. Notably, the viral spread and pathogenesis in the infected host (murine model) is driven by serine rather than cysteine proteases [10-13].
Figure 1: The entry of SARS-CoV-2 into alveolar epithelial type two cells. The S-Protein of the virus binds to ACE2 through its S1-subunit. Next, transmembrane protease TMPRSS2 cleaves the S-protein, and the internal fusion peptide combined with the S2-subunit is released. This enables the virus to undergo endocytosis. Moreover, the TMPRSS2 cleaves the ACE2 with shedding of a 13 kDa-fragment, which may augment the virus entry [9]. Bromhexine inactivates the TMPRSS2 in order to prevent the entry of the SARS-CoV-2 into the cell. View Figure 1
Therefore, the protease TMPRSS2 can serve as a target for pharmacological agents to prevent the penetration of the SARS-CoV-2 into the cell. Camostat mesylate is one of the top candidates. It has already been licensed and approved in the clinical practice in Japan and South Korea, improving chronical pancreatitis [8]. Moreover, exact results have been shown on cell cultures [8]. Furthermore, experiments on mice demonstrate, that camostat mesylate prevents the infection with the SARS-CoV [13]. Eventually, a clinical trial investigating the efficacy of camostat in 180 patients has recently been started in Denmark (National Clinical Trial Number NCT04321096).
Bromhexine Hydrochloride Inhibits the TMPRSS2 Activity
However, compared to Asia, camostat is a relatively unknown medicine in Europe. Current literature reveals an alternative drug, licensed and approved in Europe – bromhexine hydrochloride. It is an alkaloid obtained from Justicia adhatoda, well known and globally applied as a secretolytic expectorant for treatment of respiratory disorders with excessive or viscid mucus. Bromhexine stimulates the activity of the ciliated epithelium, enhances the lysosomal activity and induces the hydrolytic depolymerization of mucus protein fibers [14]. Pulmonary parenchyma concentrations of bromhexine two hours post dose are between 2,4 and 5,9 times higher than in plasma [15]. The drug shows the first order pharmacokinetics and is almost completely metabolized to diverse hydroxylated metabolites; the most famous of those is ambroxol, available also as a licensed mucolytic drug [15]. Importantly, only few adverse effects of bromhexine are described, such as nausea (reported as “common”), rash, bronchospasm, vomiting, diarrhea and fever (reported as “uncommon”). Severe complications such as Stevens – Johnson syndrome or toxic epidermal necrolysis, possibly related to the metabolite ambroxol, are casuistic (at the most 0,16 cases per million patients exposed) [16].
Similar to camostat, bromhexine inhibits the TMPRSS2 activity, thus preventing the viral entry into the cell. Bromhexine blocks the TMPRSS2 ability to activate a zymogen precursor of tissue plasminogen activator in vitro [17]. Interestingly, TMPRSS2 is blocked by much lower concentrations than required to inactivate other proteases in the cell culture [17]. Additionally, the same study demonstrates that bromhexine intraperitoneal injections in TRAMP mice (model of prostate cancer) significantly reduce the incidence of TMPRSS2-mediated lung and liver metastases [17]. Docked complex analysis of a homology model of the TMPRSS2 reveals, that bromhexine hydrochloride molecule interacts with Gln438 through a hydrogen bond and provides several additional hydrophobic interactions with other residues of the protease (the statement has not been peer-reviewed yet) [18].
However, these data cannot be simply extended to the clinical practice: Whether the concentration of bromhexine in the lung tissue of properly treated patients would be enough to prevent the virus entry through the TMPRSS2-inactivation remains unclear. As of today, a clinical trial in China is ongoing, which investigates the bromhexine efficacy, compared with other medicines (umifenovir and human interferon a2b). Approximately 60 patients with suspected and mild COVID-19-pneumonia are subjected to the investigation (NCT04273763). Another trial in Slovenia compares the efficacy of a combination of hydroxychloroquine with bromhexine with hydroxychloroquine alone in 90 hospitalized COVID-19 patients (NCT04355026). On the contrary, during another ongoing trial in Mexico health care workers receive bromhexine alone or in a combination with hydroxychloroquine to compare efficiency in preventing the development of COVID-19 (NCT04340349). Thus, the efficacy of bromhexine has already been implied by the researcher in this trial, despite the lack of clinical evidence. Obviously, bromhexine, causing almost no severe adverse effects, easily penetrating into alveolar cells, improving mucus clearance [19] and inhibiting TMPRSS2 is becoming a top prophylactic agent against COVID-19. The most doctors are familiar with bromhexine and its rare adverse effects. Thus, health care professionals in some hospitals in Russia use bromhexine hydrochloride as a prophylactic against the novel coronavirus infection in view of a shortage of personal protective equipment under hospital overload conditions (personal communication). Last, no absolute contraindications against bromhexine exist, except of a drug allergy [14,19]. Taking together, as physicians, we can fully justify this approach in the pandemic context.
Conclusions
Given the current situation, we discussed a possible application of bromhexine in our practice. We decided to prescribe bromhexine at our institution as off label use by the informed consent of patients according to the national law. Bromhexine should be prescribed for adults with confirmed or suspected COVID-19 admitted to the ICU in order to prevent a further deterioration. Whether this strategy could be applied to hospitalized patients who do not require intensive care remains unresolved. Due to the mechanism of action, we doubt on the bromhexine decisive effect on advanced COVID-19 lung dysfunction. We predict that its effect is limited to slight and middle severe disease forms. Accordingly, we highly recommend to start the therapy with bromhexine hydrochloride immediately as a patient shows an airway infection or fever symptoms and proceed with it till an improvement, intolerance signs or a doctor’s decision to withdraw. Moreover, we propose prophylactic bromhexine doses (8 mg oral administration thrice a day) for high risk groups including health care workers and older people. Although two recent publications propose the same approach [20,21], it has not been taken into consideration by experts or discussed widely yet. Obviously, a practical implementation of the suggested method requires regular reappraisals according to the new evidence. In parallel, we suggest a randomized placebo-controlled clinical trial to ascertain the real clinical efficacy of bromhexine both as prophylactic and in different stages of COVID-19. In conclusion, as long as no compelling evidence of ineffectiveness of the drug or better available alternative presents, bromhexine can serve as a major cure during the COVID-19 pandemic.
References
Yang X, Yu Y, Xu J, Shu H, Xia J, et al. (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single centered, retrospective, observational study. Lancet Respir Med 8: 475-481.
Namendy-Silva SA (2020) Respiratory support for patients with COVID-19 infection. Lancet Respir Med 8: e18.
Shetty R, Ghosh A, Honavar SG, Khamar P, Sethu S (2020) Therapeutic opportunities to manage COVID-19/SARS-CoV-2 infection: Present and future. Indian J Ophthalmol 68: 693-702.
Glowacka I, Bertram S, Müller MA, Allen P, Soilleux E, et al. (2011) Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol 85: 4122-4134.
Matsuyama S, Nagata N, Shirato K, Kawase M, Takeda M, et al. (2010) Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol 84: 12658- 12664.
Shulla A, Heald-Sargent T, Subramanya G, Zhao J, Perlman S, et al. (2011) A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol 85: 873- 882.
Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, et al. (2005) Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci USA 102: 11876-11881.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271-280.
Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, et al. (2014) TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 88: 1293-1307.
Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, et al. (2019) TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol 93: e01815-e01818.
Shirato K, Kanou K, Kawase M, Matsuyama S (2016) Clinical isolates of human coronavirus 229E bypass the endosome for cell entry. J Virol 91: e01387-e01416.
Shirato K, Kawase M, Matsuyama S (2018) Wild type human coronaviruses prefer cell-surface TMPRSS2 to endosomal cathepsins for cell entry. Virology 517: 9-15.
Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R Jr, et al. (2015) Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res 116: 76-84.
Bhagat A, Rachana R (2018) Bromhexine: A comprehensive review. Int J Bio Med Res 9: 6455-6459.
(2016) Bisolvon oral solution, summary of product characteristics, Health Products Regulatory Authority, Ireland.
(2015) Ambroxol and bromhexine containing medicinal products. EMA/PRAC.
Lucas JM, Heinlein C, Kim T, Hernandez SA, Malik MS, et al. (2014) The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov 4: 1310-1325.
Sonawane K, Barale SS, Dhanavade MJ, Waghmare SR, Nadaf NH, et al. (2020) Homology modelling and docking studies of TMPRSS2 with experimentally known inhibitors camostat mesylate, nafamostat and bromhexine hydrochloride to control SARS-Coronaviris-2.
Zanasi A, Mazzolini M, Kantar A (2017) A reappraisal of the mucoactive activity and clinical efficacy of bromhexine. Multidiscip Respir Med 12: 7.
Depfenhart M, Lemperle G, Meyer M, Rautenbach M, Bertossi D, et al. (2020) SARS-CoV-2 Prophylactic and Treatment; A counter argument against the sole use of chloroquine.
Maggio R, Corsini GU (2020) Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection. Prarmacol Res 157: 104837.
Citation
Stepanov D, Lierz P (2020) Bromhexine Hydrochloride: Potential Approach to Prevent or Treat Early Stage Novel 2019 Coronavirus Disease. J Infect Dis Epidemiol 6:135. doi.org/10.23937/2474-3658/1510135
Post time: Jun-23-2022